Moderna Vaccine Wikipedia
Messenger RNA vaccinesalso called mRNA vaccinesare some of the first COVID-19 vaccines authorized for use in the United States. Beim Impfstoff von BiontechPfizer bzw.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/WTA2LC6UFVNXPGX3DRKWZKXV5I.jpg)
New Study To Test Moderna Vaccine In Transmission Prevention Among College Students Reuters
Learn about safety data efficacy and clinical trial demographics.
Moderna vaccine wikipedia. Juli 2021 für den Impfabstand. Modalities may focus on different diseases within one therapeutic area - or across therapeutic areas - allowing us to potentially use one technological solution to create medicines for many diseases. Learn about the science of mRNA our platform our research and our development engine.
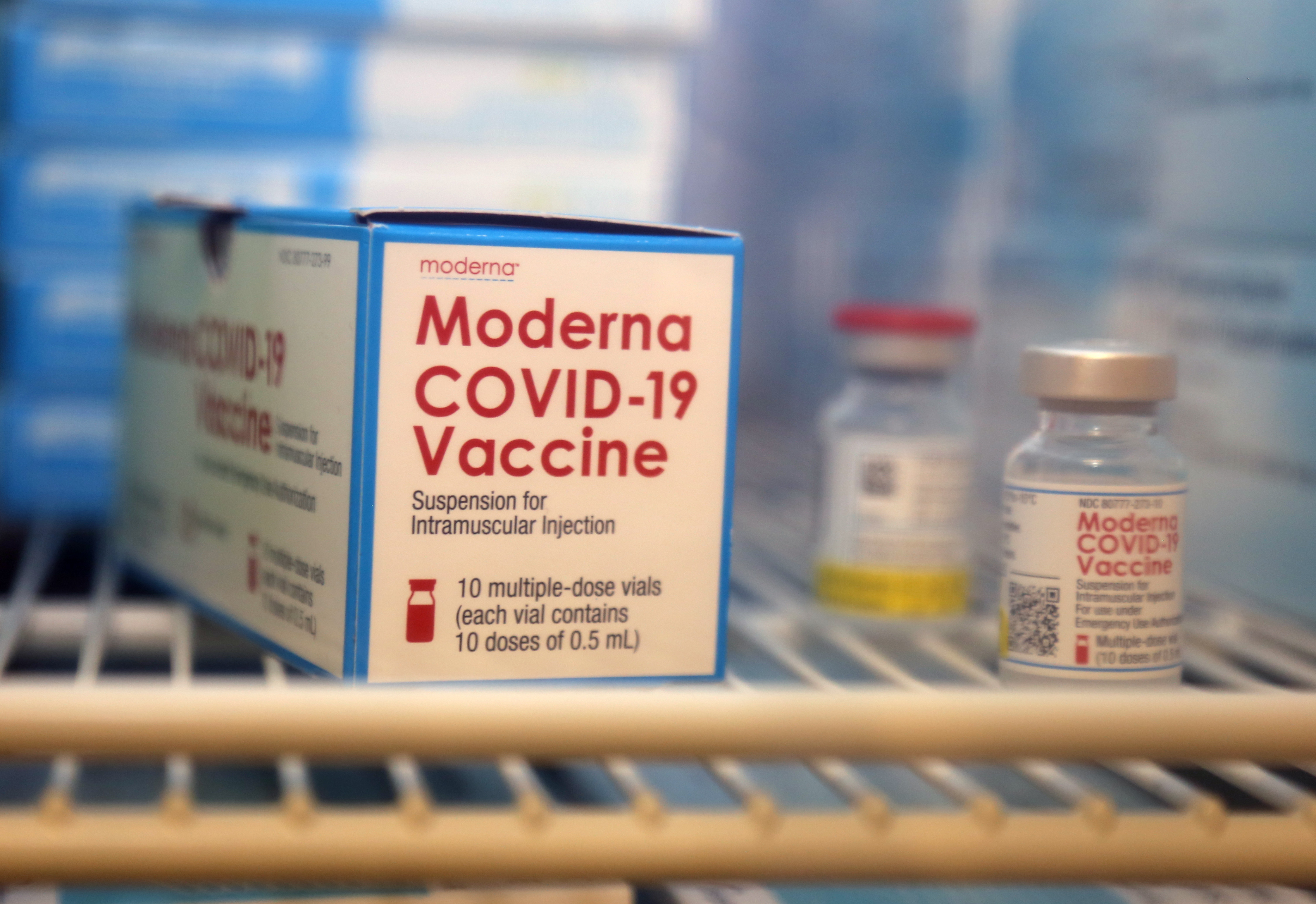
Fact Sheet for Healthcare Providers Administering Vaccine. Food and Drug Administration announced two revisions regarding the number of doses per vial available for the Moderna COVID-19 Vaccine. The vaccine was then studied in.
FDA Makes Two Revisions to Moderna COVID-19 Vaccine Emergency Use Authorization to Help Increase the Number of Vaccine Doses Available Today the US. Learn about mRNA science our. The Moderna COVID-19 vaccine also known as Spikevax is an mRNA vaccine produced by the American company Moderna the US.
He became CEO of Moderna in 2011 and owns a roughly 8 stake in the publicly. It is used in people aged 18 years and older to provide protection against infection by. Moderna betrug der Impfabstand in den Impfzentren entsprechend der Empfehlung der STIKO für längere Zeit 6 Wochen während Hausärzte den Impfstoff von BiontechPfizer dem Herstellervorschlag folgend auch im Abstand von 3 bis 4 Wochen impften.
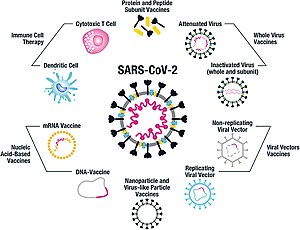
New Approach to Vaccines. Information about the Moderna COVID-19 vaccine including name manufacturer type of vaccine number of shots how it is given and links to ingredient information. In July 2020 more than 150 vaccines were being developed in different laboratories.
Two mRNA vaccines one by Pfizer and one by Moderna were approved late in 2020. For the mRNA vaccine page on Wikipedia for instance here is the history page on which changes are tracked. Biomedical Advanced Research and Development Authority and the Coalition for Epidemic Preparedness Innovations.
Or you can look at the upper right hand corner for the View History link Checking this history page out I quickly found that over the last month or so there had been a rather vigorous discussion over changing the text. Learn about safety data efficacy and clinical trial demographics. The Moderna COVID-19 vaccine is an mRNA vaccine that requires 2 shots 28 days apart.
MRNA vaccines are a new type of. Learn more about getting your vaccine. Januar 2020 meddelte Moderna om udviklingen af en vaccine til hæmning af COVID-19 coronavirus.
Moderna has developed a COVID19 vaccine based on the science of mRNA an area of focus for Moderna since 2010. If you receive one dose of the Moderna COVID-19 vaccine you should receive a second dose of this same vaccine 1 month later. Moderna - Transformative Medicines - mRNA Science.
Platform research and development. The Moderna mRNA-1273 vaccine was authorized by the Food and Drug Administration for emergency use during the ongoing COVID-19 pandemic in December 20201 Out of 15185 participants in the phase III Coronavirus Efficacy COVE trials who received at least one dose of the Moderna vaccine 228 15 reported hypersensitivity adverse events. The Moderna COVID-19 vaccine will be given to you as an injection into the muscle.
A COVID-19 vaccine is any of the vaccines used against COVID-19 a disease caused by the SARS-CoV-2 virus. Modernas teknologi er en messenger RNA-forbindelse compound kaldet mRNA-1273 som hæmmer SARS-CoV-2 der koder for en form af virussens spike-proteinVaccinekandidaten er af den RNA-baserede vaccinetype og udvikles i et samarbejde. The first thing that I note is that although.
Both are over 90 effective. National Institute of Allergy and Infectious Diseases the US. A few vaccines also went into use in early 2021.
Using knowledge of mRNA science based on 10 years of research findings Moderna developed a vaccine designed to prevent COVID19. Aug 19 2021 The Moderna COVID-19 vaccine is an mRNA vaccine that requires 2 shots 28 days apart. Die aktuelle Empfehlung der STIKO Stand 1.
We refer to a modality as a family of potential mRNA medicines consistent both in design and how and where they are delivered in the body. Stéphane Bancel is the CEO of Cambridge Massachusetts-based biotech firm Moderna known for its Covid-19 vaccine. The Moderna COVID 19 vaccine codenamed mRNA-1273 is a COVID-19 vaccine developed by Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA.
Discover mRNA technology a new approach to medicine. If you received a Pfizer-BioNTech or Moderna COVID-19 vaccine you should get the same product for your second shot. For most individuals the Moderna COVID-19 vaccine vaccination series is 2 doses given 1 month apart.

How The U S Government Bolstered Moderna S Covid 19 Vaccine Candidate Drug Discovery And Development
Covid 19 Vaccine Resources For Extension Education Professionals Nc State Extension

Moderna Covid 19 Vaccine Wikipedia
Covid 19 Chinese Vaccine Successful In Mid Stage Trials Bbc News
Ema Entscheid Moderna Impfstoff Nun Auch Fur Teenager Zugelassen Kurier At

Moderna Covid 19 Vaccine Wikipedia

Sputnik V Covid 19 Vaccine Wikipedia

Pfizer Biontech Covid 19 Vaccine Wikipedia

File Covid Vaccine 41 50753217957 Cropped Jpg Wikimedia Commons

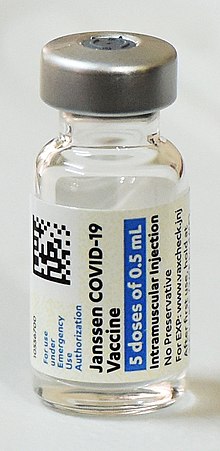
Covid Vaccines Johnson Johnson S Shot Authorized By F D A The New York Times

The Doomsday Prophecy Of Dr Geert Vanden Bossche Office For Science And Society Mcgill University

Oxford Astrazeneca Covid 19 Vaccine Wikipedia
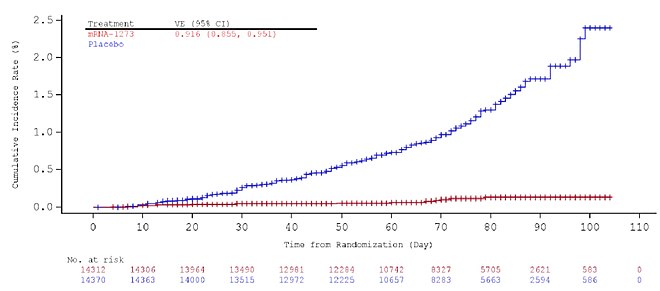
Moderna Covid 19 Vaccine Wikipedia